Background: Myelofibrosis (MF) is a BCR-ABL1-negative myeloproliferative neoplasm (MPN) characterized by progressive bone marrow fibrosis, splenomegaly, and cytopenias due to impaired hematopoiesis (Passamonti and Mora. Blood 2022). Despite the availability of comprehensive national and international guidelines for diagnosing and managing MF, gaps remain in translating these guidelines into clinical practice, particularly for patients with cytopenias, non-response or intolerance to Janus kinase (JAK) inhibitor treatment, and those ineligible for clinical trial enrollment. Consequently, an international expert consensus group was established with the objective of augmenting the existing guidance.
Objectives: To develop a set of evidence- and consensus-based recommendations for managing MF in routine clinical practice, with a focus on patients with cytopenias, including practical strategies and tools to support clinicians.
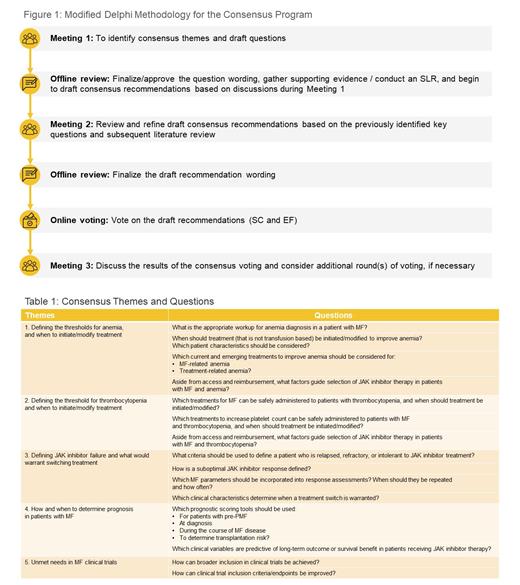
Methods : Modified Delphi methodology was utilized to achieve consensus (Figure 1). A Steering Committee (SC) of 9 expert hematologists (the authors) was established. During an initial meeting, the SC proposed 25 clinical questions that addressed key issues across 5 consensus themes: 1) Defining the thresholds for anemia and when to initiate/modify treatment; 2) defining the threshold for thrombocytopenia and when to initiate/modify treatment; 3) defining JAK inhibitor failure and what would warrant switching treatment; 4) how and when to determine prognosis in patients with MF; and, 5) unmet needs in MF clinical trials. An extended faculty (EF) was then enlisted, comprising hematologists and patient advocacy groups, who voted on the importance of the questions to address. The 15 highest scoring questions were selected for the consensus program (Table 1). To gather scientific evidence around the questions, a systematic literature review (SLR) was conducted using the PubMed and Embase databases, adhering to a PICO (Population, Intervention, Comparison, Outcome) framework. In a subsequent meeting, recommendations were formulated to address the questions using evidence from the SLR and the expert clinical experience of the SC. An online voting platform was then used for both the SC and EF to provide an agreement score for each recommendation. Consensus was achieved when 75% of the respondents agreed within the range of 7-9 on a 9-point scale (1=strongly disagree, 9=strongly agree).
Results: Consensus was achieved among voters (hematologists [n=29] and patients [n=9] from Europe, the United States, Canada, Australia, and Israel) for all 15 recommendations. Recommendations in theme 1 emphasize the importance of comprehensive evaluation, exclusion of other causes of anemia, therapy dose optimization, and consideration of additional treatments for managing anemia in patients with MF. Recommendations in theme 2 highlight the complexity of managing splenomegaly, symptoms, and anemia in patients with low platelet counts, and factors guiding therapy selection for these patients. Theme 3 recommendations discuss criteria used to determine JAK inhibitor failure, including relapse, refractoriness, or intolerance, and guidance for distinguishing between these. In theme 4, recommendations address the use of validated prognostic scores at diagnosis and during the disease course, and transplantation risk assessment. The limitations and appropriate utilization of these scores are emphasized, and the need to develop prognostic scores in pre-primary MF is highlighted. Theme 5 recommendations cover the importance of addressing unmet needs in MF clinical trials, and include striving for inclusivity by removing barriers to the participation of underserved patient populations and focusing efforts on validating additional endpoints beyond traditional measures.
Conclusions: An international panel of physicians with expertise in MF, together with a diverse EF, was able to achieve a high level of consensus across a wide range of critical gaps in MF management. These recommendations provide a valuable framework to support clinicians in optimizing care for patients with MF.
OffLabel Disclosure:
Harrison:Morphosys: Honoraria, Speakers Bureau; AOP: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Galecto: Honoraria, Speakers Bureau; CTI: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Bose:Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding; GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding. Ellis:GSK, BMS: Other: Advisory board; Gad Medical: Research Funding, Speakers Bureau; Novartis: Other: Advisory board, Speakers Bureau; GSK: Honoraria. Gupta:BMS Celgene, Roche, AbbVie, Pfizer, Sierra Oncology, CTI Biopharma, GSK: Other: Participation on a Data Safety Monitoring Board or Advisory Board; GSK: Other: Travel to EHA 2023 for invited talk at GSK sponsored MPN education session ; Novartis, BMS Celgene, SMP Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy; BMS, Celgene, Roche, Abb Vie, Pfizer, Sierra Oncology, CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis, BMS Celgene, GSK: Honoraria; Novartis, BMS Celgene, Sierra Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy. Kiladjian:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, AOP Health, Bristol-Myers Squibb, GlaxoSmithKline, Incyte, Novartis, Pharmaessentia.: Consultancy. Mascarenhas:Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; GSK: Honoraria; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy. Mathews:GSK: Honoraria. Passamonti:Roche: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Novartis, GSK, Bristol Myers Squibb/Celgene, Sierra Oncology, AbbVie, Janssen, Roche, AOP Orphan, Karyopharm, Kyowa Kirin, MEI, Sumitomo: Honoraria; Abbvie: Consultancy, Honoraria. Koschmieder:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Squibb: Consultancy, Honoraria; CTI Biopharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; RWTH Aachen University: Patents & Royalties: patent issued for a BET inhibitor; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Karthos: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Imago Bioscience: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; Geron, Janssen, AOP Pharma, Novartis: Research Funding; Pfizer, Incyte, Ariad, Novartis, AOP Pharma, Bristol Myers Squibb, Celgene, Geron, Janssen, CTI BioPharma, Roche, Bayer, GSK, Protagonist, MPN Hub, Bedrock, PharmaEssentia: Consultancy; Novartis, BMS/Celgene, Pfizer, Incyte, AOP Orphan, GSK, AbbVie, MPN Hub, Bedrock, iOMEDICO: Honoraria; Alexion, Novartis, Bristol Myers Squibb, Incyte, AOP Pharma, CTI BioPharma, Pfizer, Celgene, Janssen, Geron, Roche, AbbVie, GSK, Sierra Oncology, Kartos, Imago Biosciences, MSD, iOMEDICO: Other: Travel/accommodation support; RWTH Aachen University: Patents & Royalties: BET inhibitor; Pfizer, Incyte, Ariad, Novartis, AOP Pharma, BMS, Celgene, Geron, Janssen, CTI BioPharma, Roche, Bayer, GSK, Sierra Oncology, AbbVie, Protagonist, PharmaEssentia: Other: Advisory board; Protagonist: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; GSK: Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support; AOP Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support, Research Funding; Geron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel/accommodation support, Research Funding.
Danazol: synthetic attenuated androgen. Off-label use: treatment of MF-related anemia and thrombocytopenia. Erythropoietin-stimulating agents (ESAs): recombinant versions of erythropoietin (EPO). Off-label use: treatment of MF-related anemia. Luspatercept: recombinant fusion protein. Off-label use: treatment of MF-related anemia. Immunomodulatory drugs (IMiD): cereblon modulators, including thalidomide and its analogues. Off-label use: treatment of MF-related and thrombocytopenia. Fedratinib: JAK2 inhibitor. Off-label use: treatment of MF in patients with platelet counts <50x10^9/l. Ruxolitinib: JAK1/2 inhibitor. Off-label use: treatment of MF in patients with platelet counts <50x10^9/l. Corticosteroids: synthetic steroid hormones. Off-label use: treatment of MF-related thrombocytopenia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal